
About
Imperative Care
Who We Are
With patients as our North Star, we develop innovations that deliver a real impact for patients and their families, with a focus on making these technologies available more broadly.
With three commercially available product portfolios that address specific gaps in treatment and care of stroke and vascular disease, and more programs underway, Imperative Care is committed to rapid innovation across the entire patient journey.
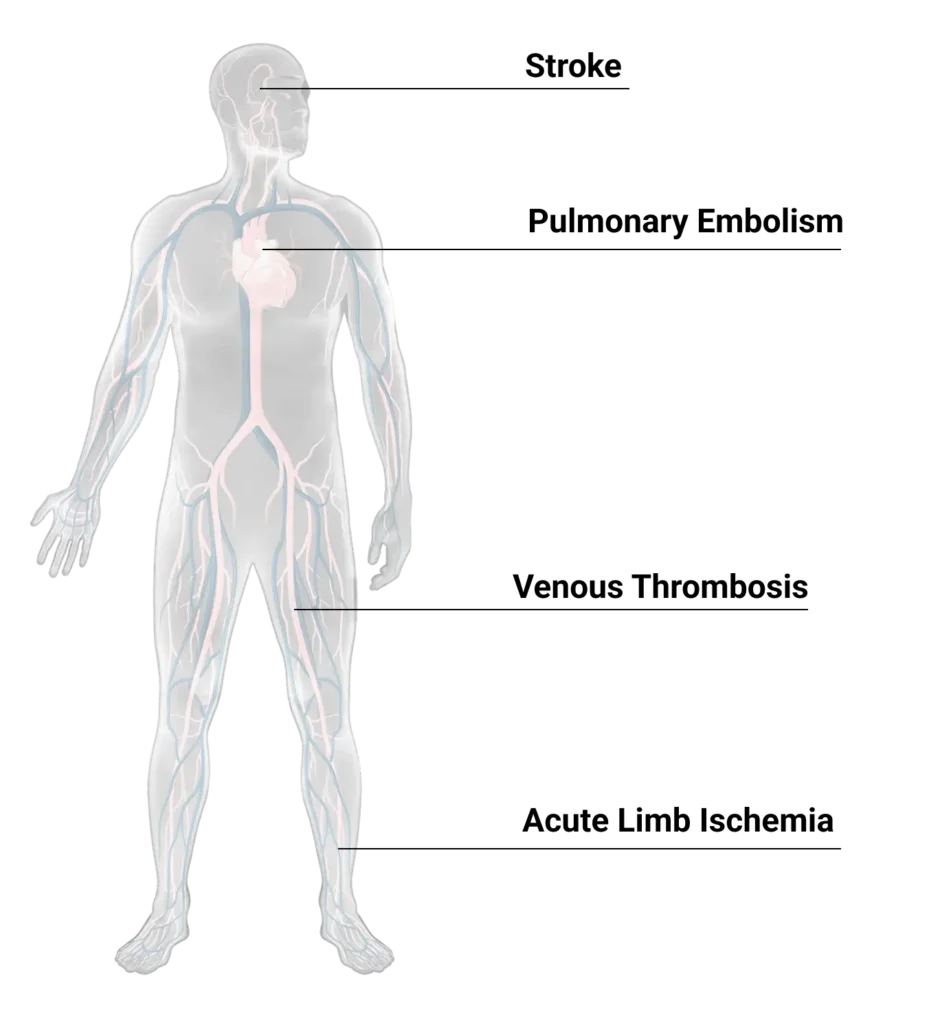

Innovating Across the Entire Patient Experience
Our vision is grander than a singular technology solution. By bringing together dedicated product portfolios – each focused on addressing a specific gap in the care continuum – under one collective vision, we are better positioned to expand and improve patient care.
Driven By Urgency
When a blood clot blocks a vital vessel, time is of the essence. Whether it’s within minutes, hours or days, the patient will suffer life-changing damage if the clot isn’t removed quickly. That’s why we’re engineering solutions to give patients the best chance at a full recovery and restored independence.
Every 40 Seconds
someone in the United States has a stroke.1
1 https://www.cdc.gov/stroke/data-research/facts-stats/index.html
Every 6 Minutes
one person dies from a blood clot in the U.S.2
2 https://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html
A Future Built on Innovation
We know that the future is dependent on the innovations we invest in today.
Our Core Tenents
Our Founding Story
Our story began in 2015, when serial medtech entrepreneur Fred Khosravi and cofounded Imperative Care with the late L. Nelson (Nick) Hopkins, M.D. F.A.C.S., a pioneering leader in endovascular neurosurgery. After decades of successful partnership, Nick and Fred recognized the clear need for a company to focus on elevating every phase of care across the entire patient journey, and making this care accessible to more people. They founded Imperative Care with a singular focus on stroke, which has since expanded to include other devastating vascular diseases.



